Temperature
Temperature is a physical property of matter that quantitatively expresses hot and cold. Temperature is measured with a thermometer.

Thermal vibration of a segment of protein alpha helix: The amplitude of the vibrations increases with temperature.
Temperature is the manifestation of thermal energy, present in all matter, which is the source of the occurrence of heat, a flow of energy, when a body is in contact with another that is colder.
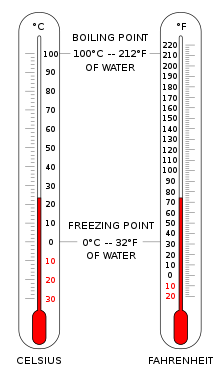
Thermometers are calibrated in various temperature scales that historically have used various reference points and thermometric substances for definition. The most common scales are the Celsius scale (formerly called centigrade), denoted °C, the Fahrenheit scale(denoted °F), and the Kelvin scale (denoted K), the latter of which is predominantly used for scientific purposes by conventions of the International System of Units (SI).
When a body has no macroscopic chemical reactions or flows of matter or energy, it is said to be in its own internal state of thermodynamic equilibrium. Its temperature is uniform in space and unchanging in time. The lowest theoretical temperature is absolute zero, at which no more thermal energy can be extracted from a body. Experimentally, it can only be approached very closely, but not reached, which is recognized in the third law of thermodynamics.
Temperature is important in all fields of natural science, including physics, chemistry, Earth science, medicine, and biology, as well as most aspects of daily life



Comments
Post a Comment